DiOC6(3) has been a useful fluorescent dye for staining the ER. It is a positively charged molecule that permeates through the plasma membrane. At low concentrations, it accumulates in mitochondria due to their large negative membrane potential. At higher concentrations, the dye stains other membranes, including the ER. In fixed cells, the dye appears to stain all intracellular membranes.
DiOC6(3) is useful only in cells where the ER structure can easily be distinguished from other organelles. In the thin peripheral regions of many cultured cells, the ER forms a single-layered network that can be clearly identified. In the thicker regions of cells, where the ER is three dimensional and where many other organelles are present, DiOC6(3) is not very useful.
Laboratory ExercisesNotes
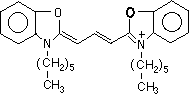
Molecular structure of DiOC6(3)
The full name of DiOC6(3) is dihexaoxacarbocyanine iodide. Fluorescence
is due to the resonant bond structure in the connecting chain and part
of the cyclic groups. A positive charge is delocalized over this resonant
bond structure. Because of this structure, the molecule is planar.
Nomenclature of cyanine dyes
The general form of the cyanine dyes is DiXCy(z). "Di"
refers to the fact that the molecule has two similar halves. X refers to
the atom or chemical group at one of the corners. The three commonly available
groups are O (oxygen), S (sulfur), and I (isopropyl). Cy refers to the
length of the two carbon (alkyl) chains on the molecule. Hydrophobicity
is mainly determined by the length of these C chains. Up to 6 or possible
longer, the dyes are water soluble, whereas 12 or higher are water insoluble.
C chains in between are not commercially available, perhaps because they
are difficult to synthesize. Lastly, z refers to the number of carbons
in the chain between the two halves of the molecule. This connecting chain
is an alternating double bond structure, ie, it is a resonant structure;
z is always an odd number. Higher z means longer wave length fluorescence.
The chemical group ("X") also has an effect on the fluorescence
wavelength because of its effect on electrons in the resonant energy structure.
DiOC6(3) should technically be written with the 6 as a subscript. In the cell biology literaure, it has been referred to as DiOC6 or DiOC or even DECC. Another molecule, DiOC18(3) is very often referred to as "DiO" - this molecule has different staining characteristics, as it has two C18 chains and is insoluble in water.
An essay on the ER organization in the peripheral regions of fibroblastsMicrotubules and the ER
When nocodazole or other microtubule depolymerizing drugs are put onto
cultured cells, the ER undergoes a slow retraction towards the cell center.
This leaves the peripheral regions devoid of ER. Because of this result,
we became interested in co-localizing microtubules and the ER (Terasaki
et al., 1986). Since immunofluorescence requires permeabilization, which
destroys DiOC6(3) staining, it was necessary to stain the ER first, take
photographs, then label the microtubules and find the same cells and re-photograph
them.
Silicon rubber sheet.
order from:
Kentron-Ronsil Division
1 Polymer Place
Blackstone, VA 23824
phone: 804 292 1600
calendared sheet, 0.03 inches thick, 12 inches wide
They generally ask that you buy $100 worth at $6/foot.
Fixation, fixatives.
Glutaraldehyde has five carbons with two aldehyde groups at each end. These
groups are thought to cross link free amino groups in the cell, causing
"fixation". Formaldehyde is a smaller molecule with one aldehyde
group. The mechanism of fixation is not as well understood.
Glutaraldehyde was discovered as a fixative by Sabatini et al. in the early
1960's. It is still the standard fixative for electron microscopy, and
is superior to formaldehyde fixation. Typically, tissues are fixed in 1-2.5%
glutaraldehyde for an hour.
Glutaraldehyde is not as commonly used as formaldehyde for fluorescence
microscopy. This is because glutaraldehyde causes autofluorescence. Autofluorescence
develops slowly with time, and depends on the concentration of glutaraldehyde
used. It is sometimes possible to use low concentrations and rapid procedures
in order to observe fluorescence labeling before the autofluorescence swamps
out the signal. Glutaraldehyde fixes cultured cells more rapidly than tissues,
so it is possible to use lower concentrations and briefer periods to fix
them than tissues. I have sometimes used as low as 0.025% glutaraldehyde
for 5 min as fixation of cultured cells. This barely fixes cells, and appears
to be not quite enough to produce autofluorescence.
The problem of whether the dye was staining the ER was an interesting one. The immunofluorescence staining of the early 1980's didn't show a network in cultured cells. But when M. Terasaki looked in Ham's Histology to find out how the ER was discovered, he found out that Keith Porter had discovered the ER as an identical network in cultured cells. In the 1940's, Porter was trying to look at cells by electron microscopy before the invention of the microtome. He had the idea of growing cultured cells on EM grids, and looking through the thin peripheral regions of the cells without sectioning. It was there that he saw a reticular network in osmium fixed cells. Since this network was more abundant in the central regions, and less abundant in the lamellipodia, he called it the "endoplasmic reticulum", since the central region is sometimes called the endoplasm and the lamellipodia the ectoplasm. When the fluorescent dye images were shown to Porter and to others of the old Rockefeller school of electron microscopists, they immediately identified it as endoplasmic reticulum, whereas many modern cell biologists of the 80's said all sorts of other things when they first saw it! The reason why immunofluorescence images of anti-ER proteins usually did not show a network was that cells had to be permeabilized by detergents or organic solvents in order for the antibodies to have access to the ER antigens. Detergents and organic solvents not only permeabilize the plasma membrane, they also disrupt the ER membranes, and it is generally difficult to preserve the original network morphology.
Once we had learned of this history, Jin Dan Song, a visiting professor in Lan Bo Chen's lab, grew some cells on EM grids and fixed them with potassium permanganate, a fixative used primarily in the late 1950's which stains membranes very well. These produced electron microscopic images of the ER network that were more clear than the original images of osmium fixed cells. This was published in the original paper on DiOC6(3) and in a later paper (Song et al., J. Structural Biology 107: 106, 1991).
So the question of the "discovery" of DiOC6(3) is more a realization of what it stained. The dicarbocyanine dyes are artificial, man-made molecules. From my reading of their history, it appears that these type of dyes were being studied by the photographic industry in the 1930's in order to make "panchromatic" film. Silver salts are more sensitive to blue light, so that the early black and white photography did not pick up red colors as well. The panchromatic films had dyes in them which absorbed red light and activated the silver grains.
DiOC6(3) and other dicarbocyanine dyes were also used in biology prior to their use as mitochondrial stains. These dyes were used as voltage sensitive dyes in the 1970's. The dicarbocyanines were one of the "slow" dyes useful for membrane potentials that did not change rapidly (such as red blood cells - Sims et al., Biochemistry 13: 3315, 1974) compared to the fast styryl dyes used for neurons. There was even a report that dicarbocyanine dyes stained the sarcoplasmic reticulum of heart cells in 1980 (Habicht and Brune, Exp Cell Res 125: 514).
Comparison with BiP
BiP is a protein that is present in the lumen of the ER. It participates
in the folding and oligomerization processes of newly synthesized proteins
in the ER. Immunofluorescence with anti-BiP labels a network in the periphery
of cultured cells. In double labeling experiments with DiOC6(3), the network
patterns were identical (Terasaki and Reese, 1992). For these experiments, the cells were fixed with 0.025% glutaraldehyde for 5 min, stained with DiOC6(3), photographed, then put into -20 C methanol for 5 min and processed for immunofluorescence with anti-BiP.
References
Terasaki, M., Song, J., Wong, J.R., Weiss, M.J., and Chen, L.B. 1984. Localization
of endoplasmic reticulum in living and glutaraldehyde fixed cells with
fluorescent dyes. Cell 38: 101-108.
Terasaki, M., Chen, L.B., and Fujiwara, K. 1986. Microtubules and the endoplasmic
reticulum are highly interdependent structures. J. Cell Biology 103: 1557-1568.
Terasaki, M. and Reese, T.S. 1992. Characterization of endoplasmic reticulum
by co-localization of BiP and dicarbocyanine dyes. J. Cell Science 101:
315-322.
Terasaki, M. and Reese, T.S. 1994. Interactions among endoplasmic reticulum,
microtubules and retrograde movement of the cell surface. Cell Motil. and
Cytoskel. 29: 291-300.
Terasaki, M. 1989. Fluorescent labeling of endoplasmic reticulum. In
Fluorescence Microscopy of Living Cells in Culture, Methods in Cell Biology,
Vol. 29, ed. Y.L. Wang and D.L. Taylor, Acad. Press, Inc., p. 125-135.
Terasaki, M. 1993. Probes for endoplasmic reticulum. In Fluorescent probes
of living cells: A practical manual. W.T. Mason, ed. Academic Press, London
p. 120-123.
Terasaki, M. 1994. Labeling of endoplasmic reticulum with DiOC6(3). In
Cell Biology: A Laboratory Handbook, J. Celis, ed. Academic Press, Orlando,
FL, vol. 2, pp. 381-386.